Use the Terms Solvent and Solute in Describing
Syrup -sugar solute -water solvent. In a solution thee substance that gets dissolved in the solvent is called the solute.

This Lesson Involves An Exciting Experiment To Understand The Terms Dissolve Solute Elementary School Lessons Elementary School Science Science Lesson Plans
Find an answer to your question Describe the parts of a solution using the terms solvent and solute.

. We name it a homogenous mixture because the composition is uniform throughout the solution. Models of solute and solvent distribution between the mobile phase and surface-influenced stationary phase are presented and defined in terms of class. Salt is the solute that dissolves to form a saline solution in water the solvent.
Repeat this experiment using different household substances such as sugar flour etc. A solution is composed of two components as solutes and solvent. Solvent is basically the thing or substance to which we add our solute to make it a solution.
A solute is typically a solid dissolved into a liquid. A solution is the combination of the solute aka particles or stuff and the solvent aka liquid. The solvent is present in the higher amount than the solvent.
Terms in this set 21 Identify the solvent and solute in a solution made by dissolving a small quantity of baking soda in water. A homogeneous mixture consisting of a solute dissolved into a solvent. The boiling point of a solvent is usually lower than that of a solute.
Here is the comparison between solute and solvent in terms of meaning boiling point physical state dependability. Use the terms solvent and solute to describe how to. In a solution the amount of solute is less than the amount of solvent.
The solute is present in the lesser amount than the solvent. Notice how solutions are all around us. The dispersed step of a solution is known as the solute.
Water is the solvent and baking soda is the solute. Miller-Keane Encyclopedia and Dictionary of Medicine Nursing and Allied Health Seventh Edition. Solutes are solids that are able to be dissolve in a solvent.
A solution is a mixture. The material present in the smaller amount in the solution. The substance that gets dissolved in the solvent in a solution is called as the solute.
So when we dissolve solid in a liquid the solute is a solid as it is present in smaller amount and solvent is the liquid as it is present in a larger amount and the. Generally Solute is in less amount and solvent is in more amount. The substance that gets dissolved in the solvent in a solution is called as the solute.
A solution is a homogeneous mixture of two or more substances. A mixture that has more dissolved solute than is predicted by its solubility at the given temp. The part of a solution that is present in the greatest amount is called a solvent.
Once the solute dissolves into the solvent you have a solution. What Are Examples Of Solvent And Solute. Solute solvent and solution.
Identify which substance is the solute and which is the solvent. Give an example in your description. The boiling point of a solute is usually higher than that of a solvent.
The dissolved substance in a solvent. Give an example in your description. Generally in a solution the solute is the substance present in a smaller amount and the solvent is the substance present in a larger amount.
On the other hand water vapour is considered an air solute because nitrogen and oxygen are present in the gas at much higher levels of concentration. When a solute is placed in a solvent the concentrated solute begins to slowly break apart into tiny pieces. The key difference between solvent and solute is that the solute is the one to be dissolved while the solvent is responsible for dissolving it.
The molecules of the solvent began to move out of the way in order to make room for the molecules of solute. A solution is a homogeneous mixture of two or more substances. The substance that dissolves the solute in a solution is called as the solvent.
Formed one substance dissolves into another. The solution of a lpg is gas-solid the solvent mixture of hydrocarbon and the solute is butane. It is present in a lesser amount than the solvent.
The solvent is present in the higher amount than the solvent. Also the components of a solution are mainly of two types. Solūt the substance that is dissolved in a liquid solvent to form a solution.
Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solventThe solubility of a substance fundamentally depends on the physical and chemical properties of. The solute is present in the lesser amount than the solvent. The solvent is the liquid that dissolve the solute.
The substance that dissolves the solute in a solution is called as the solvent. And solutions are a very handy thing to have around. What Is The Solute And Solvent Of LPG.
Water is referred to as the universal solvent because of its ability. A mixture that contains as much solute in it as possible at a given temp. Solute Solvent.
The solute must be soluble in the solvent. Its the final product. Its the liquid that the solute is dissolved in.
A solute or more in a solvent. With your parents permission post a picture of. The material present in the larger amount in the solution.
Solute Vs Solvent. A solvent is usually a liquid. A solute is the part of a solution thatis dissolved and the solvent is what dissolves the solute in thesolution.
The solvent is the solutions medium step which disperses the solute particles. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solventThe solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature pressure and presence of other chemicals. Shock989 Shock989 01262021 Physics Middle School answered Describe the parts of a solution using the terms solvent and solute.
A simple example to illustrate the definition of solute solvent and solution is a bucket of water containing. Use the terms solvent and solute to describe how to. 2003 by Saunders an imprint of Elsevier Inc.
Solubility of a solute in a solvent depends on solute properties such as surface area of the solute.

Unit 10 Review Describe The Following Terms Solution Solvent Solute Soluble Insoluble Miscible Immiscible Homogeneous Mixtures Of 2 Or More Substances Ppt Download

Understanding Solutions Ppt Download

Solutions Homogeneous Mixture Containing Two Or More Substance Called The Solute And The Solvent Solute Substance That Is Dissolved Lower Quantity Ppt Download

Difference Between Solute And Solvent In Tabular Form

Lesson Worksheet Solubility Solutes And Solvents Nagwa

1 Ch 14 Solutions Solutions Are Homogeneous Mixtures Solute Solvent Solute Is The Dissolved Substance Seems To Disappear In The Solvent Solvent Ppt Download

Unit 10 Review Describe The Following Terms Solution Solvent Solute Soluble Insoluble Miscible Immiscible Homogeneous Mixtures Of 2 Or More Substances Ppt Download

What Is A Solution The Amount Of A Substance That Dissolves In A Given Volume Of Solvent At A Given Temperature A Solution In Which The Solvent Is Water Ppt Download

Matter Mixture Solution Solvent Solute By Katie Christiansen
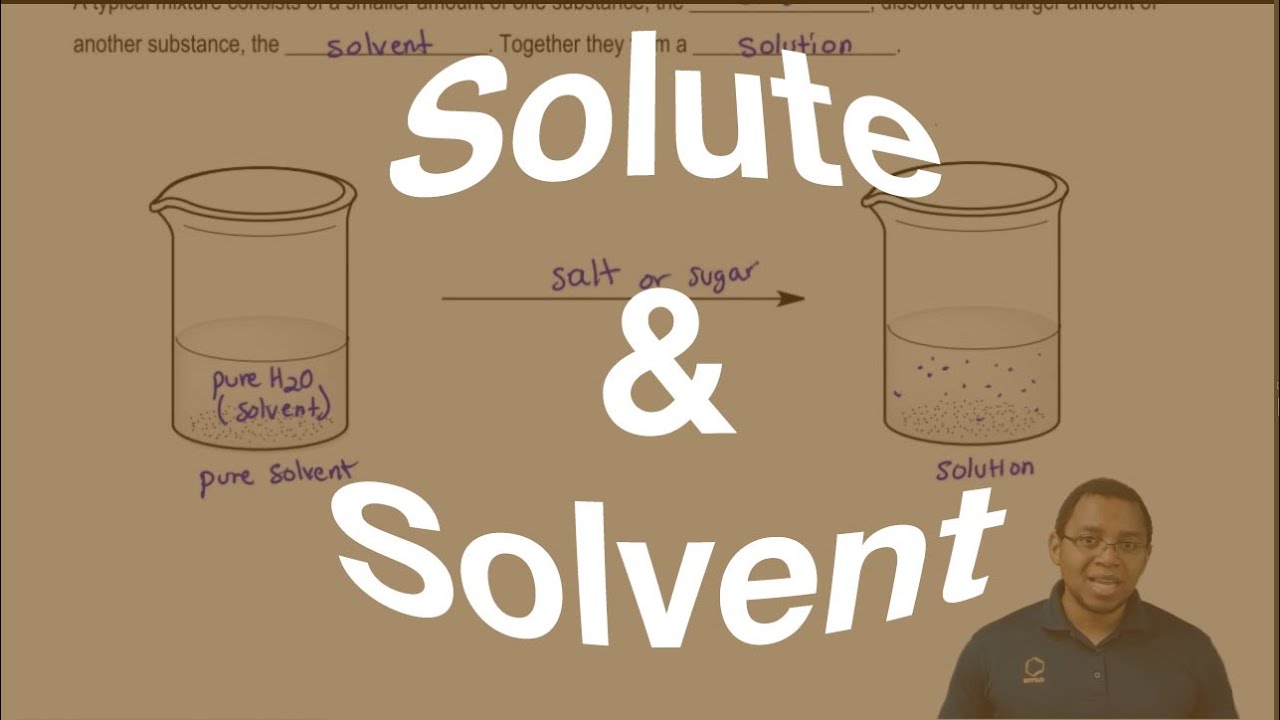
The Difference Between A Solute And Solvent Youtube

Do Now Define The Following Words Solute Solvent Solution Provide An Example Of Each Ppt Download

Learn About Solutions Science For Kids Chemistry Classroom Science For Kids Teaching Science
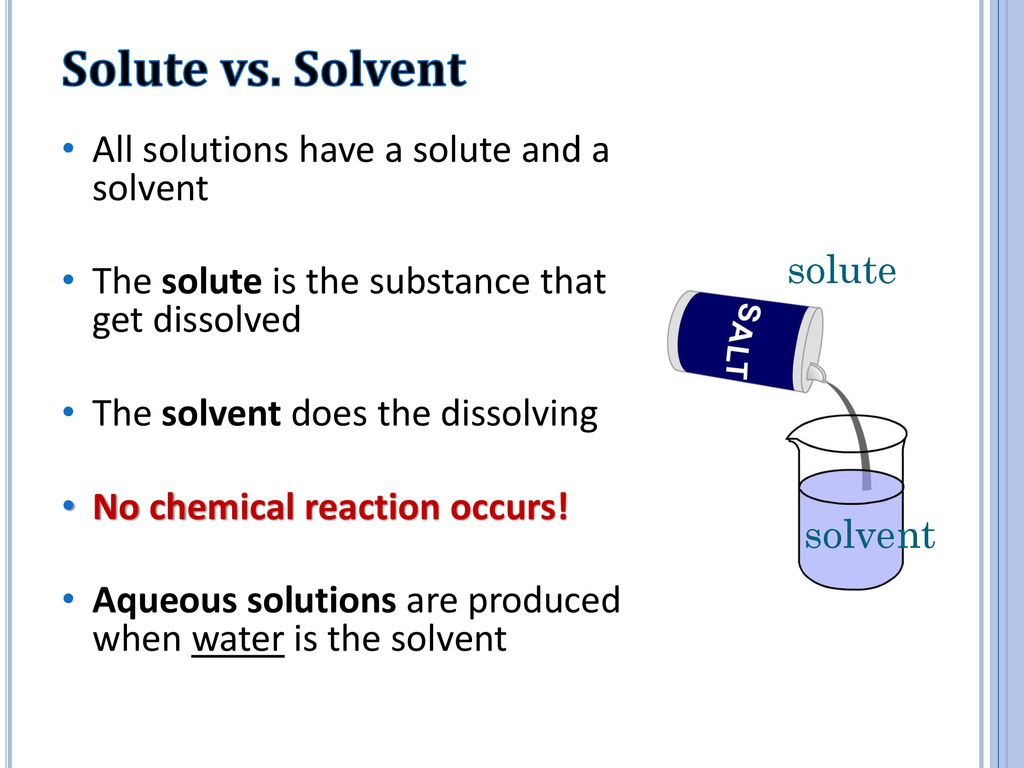
Solutions And Solubility Ppt Download
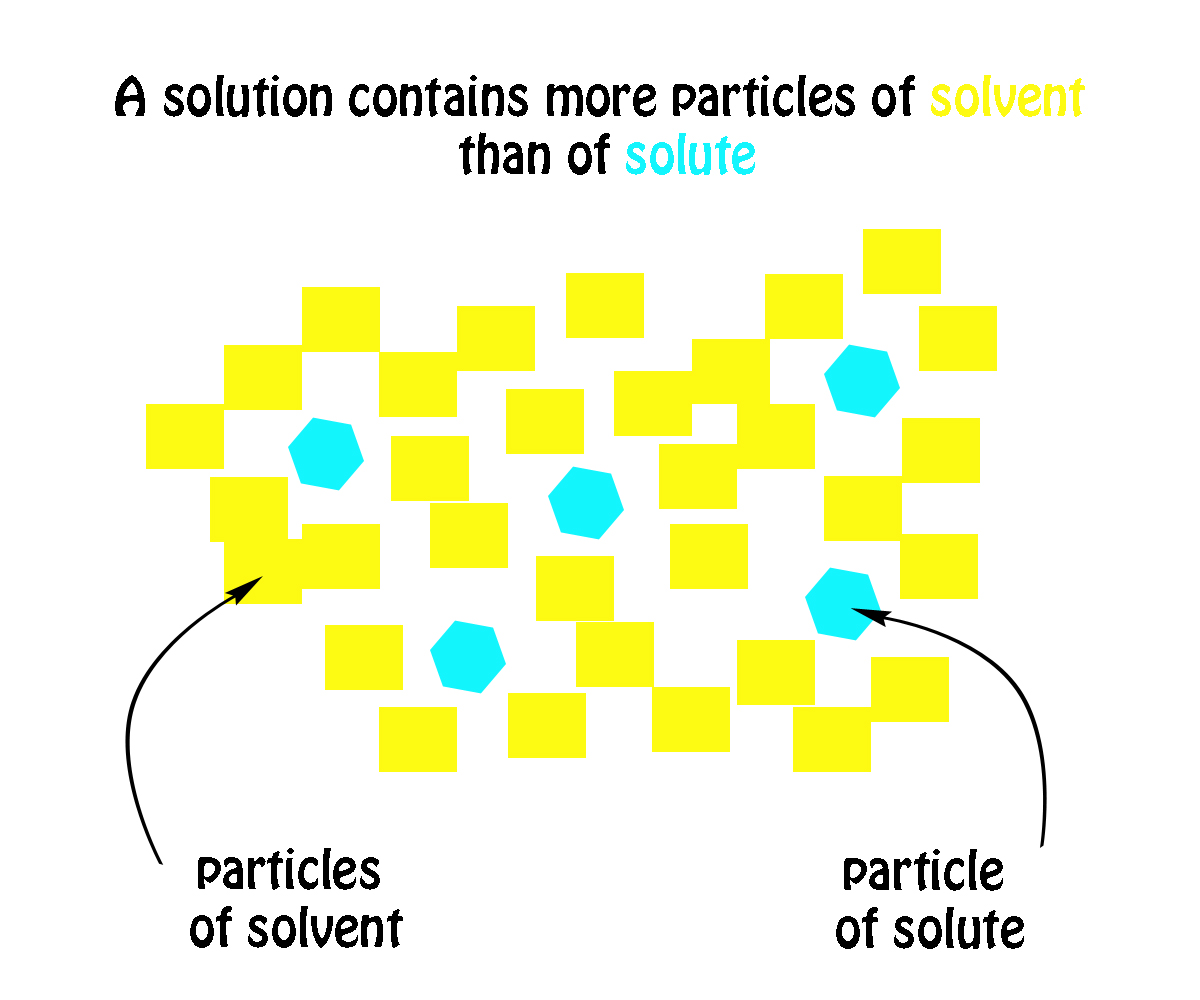
How Would You Identify The Solute And Solvent In This Solution Table Sugar C 12h 22o 11 In Water Socratic

Solutions Concentrations Of Solutions Solute Versus Solvent Solute The Substance Usually A Solid Sometime A Liquid Being Dissolved Or Broken Down Ppt Download

Mixtures Solutions Solute Vs Solvent Handout In 2022 Science Teaching Resources Teaching Science Science Worksheets

Solute Solvent And Solution Chemistry Youtube

Lennard Jones Parameters For The Solute And Solvent Particles Download Table

Solvent Solute Solutions And Solubility Matter Pure Substances Elementscompoundsmixturessolutionssuspensions Ppt Download
Comments
Post a Comment